Einstein’s Explanation of the Unexplainable
Quantum mechanics assumes the quantization of energy is what prevents electrons from falling into the nucleus of atoms. However, Classical Wave Mechanics provides another explanation base the observation that a system which is oscillating at its natural resonant frequency is one the most efficient ways to store and transfer energy between different storage modes. This combined with the law conservation of energy which tells us it can neither be created or destroyed suggests the reason why electrons do not fall into the nucleus MAY BE because the most efficient way to store their energy is in resonate systems.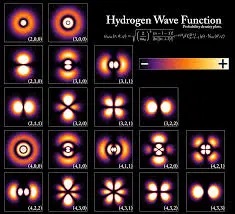
One of the core principals of quantum mechanics is that the energy of all electrons is stored in a wave defined by de Broglie’s equation ?dB = h/p.
Therefore, to verify the reason electrons do not fall into the nucleus is the law conservation of energy and not the fact that quantum mechanics tell us it is quantized one must first show how a resonate system can be created in the space around the nucleus in terms of the non-quantized properties of a wave.
Science of wave mechanics tells us the wave energy of an electron would move continuously in the space around the nucleus it is bound to. However, as mentioned earlier a system which is oscillating at its natural or harmonic of its resonant wavelength is one the most efficient ways to store energy. Therefore, the most efficient way to store it would be in a wave moving in a path where the circumference is equal to the wavelength or a harmonic of its resonate system.
However, observations and the science of wave mechanics also tells us the energy of a resonant system, such as a standing wave can only take on the discrete or quantized values associated with its fundamental or a harmonic of its fundamental frequency.
This tell us the energy of the electrons orbiting an atom MAY NOT be quantized just because quantum mechanics say they are but because the most efficient way to store their energy is in a quantized resonant system.
As was mentioned earlier energy can neither be created or destroyed therefore an electron’s energy could NEVER repeat NEVER disappear by falling into a nucleus and therefore it MUST repeat MUST be stored someplace.
Yet as was also mentioned earlier classical wave mechanics tells us the most efficient way to store energy is in resonant system such as the standing wave. This tells us the energy in each level would most likely be stored in a resonant system or standing wave that has the energy associated with that level.
Both quantum mechanics and as was shown above classical wave mechanics gives valid reasons why electrons do not fall in the nucleus. Quantum mechanics assumes they do not because their energy is quantized based ONLY on the assumption it is quantized. However, as was show above classical wave mechanics and law of conservation of energy gives another reason which are just as valid in terms of the observable properties standing waves and the fact that energy has NEVER been observed to be either created or destroyed.
Putting it another way the reason electrons do no fall into the nucleus MAY NOT be because Quantum mechanics tells us they are quantized but because observations of resonant systems and the law of conservation of energy tell us their energy can NEVER repeat NEVER be destroyed or as mentioned earlier disappear into the nucleus.
Physics is a science based on observation. Therefore, if two ideas give the same result one should give more creditability to the one which can be verified observationally instead of one that cannot.